Hydrophobic Interaction Chromatography (HIC) is a powerful method for separating proteins and biomolecules based on their surface hydrophobicity. It is performed under native conditions and is ideally suited for analyzing protein variants and aggregates without compromising biological activity.
How HIC Works
Biomolecules bind to hydrophobic ligands on the stationary phase in the presence of high salt concentrations.
Elution occurs by gradually reducing the salt concentration.
Compatible with HPLC and UHPLC systems for analytical applications.
What HIC is used for?
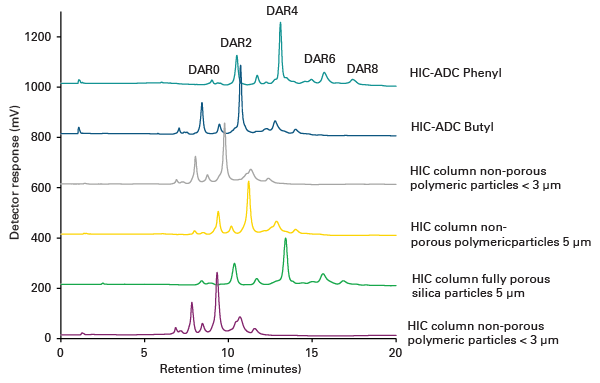
Drug-Antibody-Ratio (DAR) analysis of Antibody-Drug-Conjugates (ADCs)
Protein modifications analysis in proteins (Deamidations, Oxidations, Reductions, Sialylation…)
Protein Aggregate determination
Hydrophobicity screening in protein drug candidates
All biomolecule separations by hydrophobicity at native conditions
What Tosoh offers
Wide range of ligand chemistries for different selectivities
Columns optimized for HPLC and UHPLC performance
Proven quality for demanding biopharmaceutical analyses
Technical support and application expertise
Figure: Analysis of Drug-to-Antibody Ratios in an ADC mimic